Contract Development
Development and registration of innovative devices, software and medical products
MRC's experts cover the full range of technical and formal requirements for the development and registration of medical products. This expertise is also very valueable for non-medical products. We offer our expertise on your request and look forward to support your vision.
Further information
CE labeling and Quality Management
We support your products in all steps of their development life cycle from the concept to the production. We can also guide you through the processes required for CE marking of medical products, like risk management, application of harmonized standard, or documentation of general requirements.
MRC has established a Quality Management System in accordance with the ISO 9001 already in 1998. We have updated our processes to the new requirements of EN ISO 13485, the standard for medical products. Our operating procedures integrate the high demands of medical and other high-tech products in development & manufacturing with the force of analytical and creative engineers.
Laser & Optics
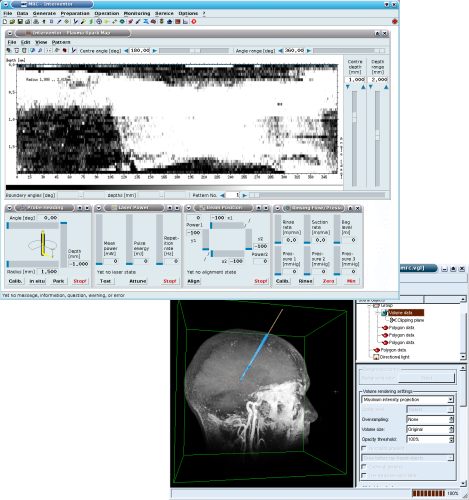
We have been working on different projects where lasers, LEDs or other light sources are required. In the medical field, we have pushed e.g. projects based on the application of ultrashort pulsed lasers for minimal-invasive tissue removal. In addition to therapeutic applications of lasers we have also developed diagnostic tools like an endoscopic confocal microscope or a plasma spark detector. For scientific and technical fields we have realized illumination devices, optical measurement systems and laser detectors.
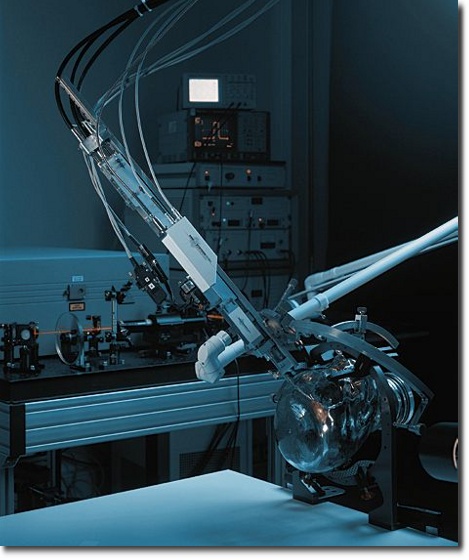
Mechanical Systems
We develop and produce mechanical systems both as stand-alone products and/or parts of integral products. The photo shows a prototype of a dynamic seat with a surface that simulates the pelvic movement in accordance with the natural human gait.
Other examples are various surgical instruments, bone implants and non-invasive medical products, but also mechanical laser components and accessories for optical setups.
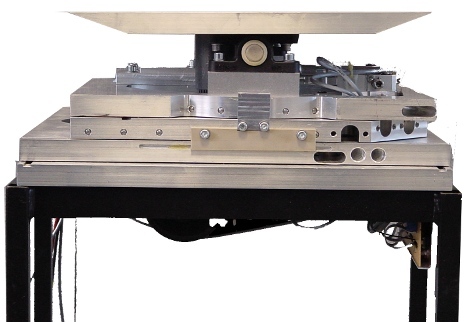
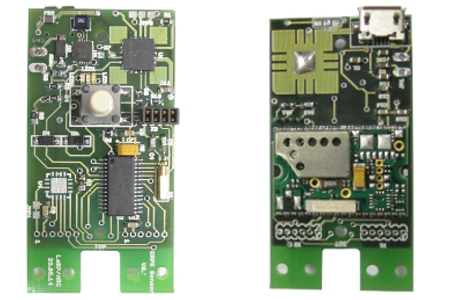
Electronic Devices
We continuously develop, verify and produce electronic devices. Some examples are opto-electronic detectors and trigger circuits, power supply systems for the use in strong magnetic fields and safety switching circuits for integrated systems. We are familiar with the relevant standards and can fulfill the most requirements for conformity testing in-house.
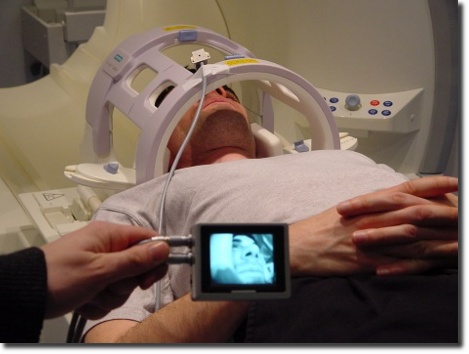
Accessories and Consulting for MRI
We have a wide experience in the development and installation of MR compatible and MR safe equipment. Our solutions fulfill the challenging mechanical and electronic requirements brought in by high magnetic field strengths and high-intensive RF fields. Examples are our own MRI-compatible products (stereotactic systems, video cameras, and Pinprick stimulators) as well as further sensory and control electronics which we created for our customers.
We support:
- the development of new products for applications in MR scanners
- the adaption of your medical equipment to MRI conditions
- your installation of MRI equipment as well as your applications in the MR
Software
With our experience from highly innovative R&D projects in the fields of radiotherapy, neurosurgery and automation we develop software and modules for different applications in medicine and technology. The projects include real-time controller software for intra-operative operation and monitoring, substantial safety mechanisms, and graphical user interfaces.
Clinical Trials
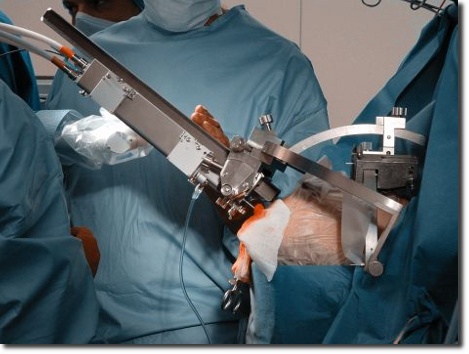
Due to the work in numerous fields of medicine our experienced staff is familiar with clinical workflows and the requirements of operation rooms. We conduct clinical trials with instruments and systems in accordance with the international standard EN ISO 14155 "Clinical investigation of medical devices for human subjects". Depending on your wishes and requirements we can perform the technical, clinical and administrative handling and also the communication tasks with users, ethics committees and public authorities.


 Deutsch
Deutsch